123 Main Street, New York, NY 10001
The Co-Dx Logix Smart ABC test kit is an in vitro diagnostic test that uses our patented Co-Primers™ technology for the qualitative detection of the RNA from the influenza A, influenza B, and SARS-CoV-2 viruses.
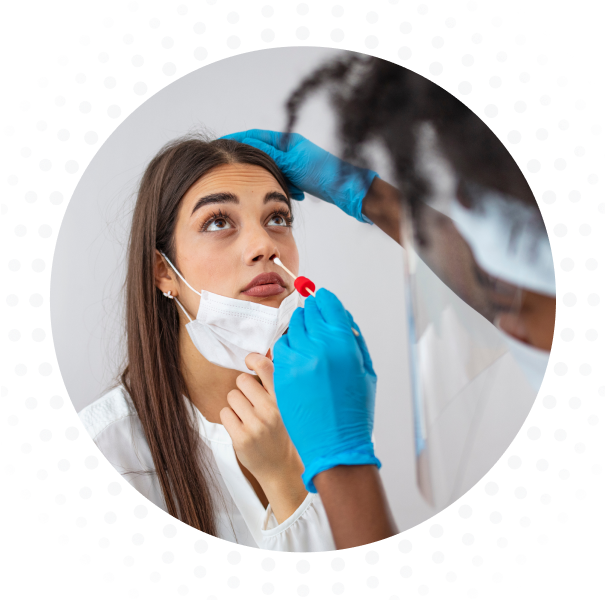
The multiplex test operates using a single step real-time reverse transcriptase PCR process in lower respiratory tract specimens (e.g. bronchoalveolar lavage, sputum, tracheal aspirate), upper respiratory tract specimens (e.g. nasopharyngeal, nasal, and oropharyngeal swabs), and saliva from individuals suspected of influenza A, influenza B, or COVID-19 and its related conditions.
Click here to read the Company’s regulatory bulletin on the ability of our suite of Co-Dx Logix Smart COVID-19 diagnostics to detect all known strains of SARS-CoV-2.
Positive results are indicative of the presence of influenza A, influenza B, and/or SARS-CoV-2 genomic material; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Many laboratories are required to report all positive results to the appropriate public health authorities.
Negative results do not preclude influenza A, influenza B, and/or SARS-CoV-2 infections and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.
The Co-Dx Logix Smart ABC test is intended for use by qualified and trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR and in vitro diagnostic procedures.
* Sensitivity based on clinical study of 30 clinical remnant SARS-CoV-2 positive samples. 15 contrived influenza A samples. 15 contrived influenza B samples and 30 negative clinical remnant samples.
Every test from Co-Dx is designed to be easy to use.
In November 2020, the Co-Dx Logix Smart ABC (influenza A/influenza B/COVID-19) test kit received CE marking approval, the principle regulatory clearance allowing the test to be sold as an in vitro diagnostic (IVD) for the diagnosis of COVID-19 in European Union states and other markets that accept a CE-IVD mark as valid regulatory approval.
Is this test FDA-approved or cleared?
No. This test is not yet approved or cleared by the U.S. Food and Drug Administration (FDA). This product is released for use as an in vitro diagnostic device, for export only and is not for sale in the United States.
Co-Diagnostics, Inc.
2401 S. Foothill Dr. Suite D
Salt Lake City, UT 84109 USA
1 (801) 438-1036
info@co-dx.com
Co-Dx, Co-Primers, Logix Smart, Co-Dx Box, PCR Home, PCR Pro, and Vector Smart, and unless otherwise specified, all other product and service names appearing in this Internet site are trademarks owned by Co-Diagnostics, Inc., its subsidiaries or affiliates. No use of any Co-Diagnostic trademark, trade name, or trade dress in this site may be made without the prior written authorization of Co-Diagnostics, except to identify the product or services of the company.
TaqMan is a trademark of Roche Molecular Systems.